Author's Information
Volz, T. 1; Lütgehetmann, M. 1,3; Dandri, M. 1,2
1. Medical Clinic, University Medic al Center Hamburg-Eppendorf, Hamburg, Germany, 2. German Centre for Infection Research (DZIF), Munich, Hamburg and Heidelberg partner sites, Germany, 3. Institute of Microbiology, Virology and Hygiene, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Experimental Procedures
Generation of human liver repopulated mice
- To generate stable infected mice with hepatotropic viruses (HBV: Hepatitis B Virus; HDV: Hepatitis Delta Virus) human liver repopulated mice are needed [1].
- Immune compromised mice (i.e. SCID: severe combined immune deficiency; ILγ: interleukin gamma knock out or RAG2: recombination activating gene 2), with liver damage (uPA: urokinase plasminogen activator or FAH: fumarylacetoacetate hydrolase), have to be transplanted intrasplenically (i.s.) with 1Mio fresh or frozen human hepatocytes [2]. These may be isolated from surgically removed organs, from a human repopulated mouse or may be commercially acquired).
- 8 weeks after transplantation the repopulation phase is typically completed and the amount of human cells in the mouse liver is stable. To confirm the successful engraftment, blood from the mouse is checked for albumin levels by a human specific ELISA test. The amount of albumin in the sera is directly correlated to the total amount of human cells inside the mouse liver.
Note: Different batches of human donor hepatocytes may produce different amounts of albumin. For the human albumin Elisa the typical dilution of mouse sera is 1:40,000 although this may need to be adapted to be in the linear range of the test.
Source of viral inoculum
- fresh or frozen viral preparations can be used as HBV-or HDV-inoculum
- Cell culture derived (ccd) inoculum (virions can be prepared from supernatant via UC centrifugation or heparin based columns)
- patient derived sera infected with either HBV alone or coinfected with HDV
- Mixtures of patient sera with ccd virions or ccd HBV with ccd HDV
- infectious mouse sera obtained by collecting blood from a previously infected humanized mouse (sera from capillaries used for i.o. blood draw or from a sacrificed mouse)
- The MOI depends on the amount of inoculum and repopulation level in each mouse (e.g. when using 10 Mio viral copies in 100µl inoculum and the mouse harbours 10 Mio human hepatocytes that would result in a MOI=1)
- Lower amount of virus genome equivalents can be used [3], in which case longer time may be needed to achieve stable infection. [4]
Note: It is recommended to aliquot the inoculum to avoid freeze thaw cycles resulting in loss of intact viruses. Human sera can be used undiluted but should be tested for the presence of possible co-infections. For dilution of the inoculum, sterile PBS or 0.9% NaCl can be used. To maximize viral input when the source has a very low viral concentration, repeated infections on consecutive days can be considered.
Route of infection
- As routes for infection the following possibilities are applicable: i.p. max. volume 150-200µl, intra venous (i.v.) or i.o. in a max. volume of 50-100µl
Blood clean up and titer measurement
- After clogging for 30min at room temperature (RT) the blood is centrifuged at 10000rpm for 10min in a table-top centrifuge. The clear upper part (sera) contains the intact viruses containing the viral DNA/RNA from the mouse (5-20µl of sera taken from capillary blood take or up to 500µl of sera from a sacrificed mouse).
- 5µl is afterwards cleaned up by a column based kit (Qiamp Minelute). To keep the detection limit as low as possible viral nucleic acids should be eluted in a minimal amount of H20 (25µl) and the maximal available volume of sera can be used if needed as input for clean-up. Ideally sera from a non-infected mouse is used as negative control in parallel.
- Real-time PCR is carried out with running a plasmid standard of known viral copies as a reference. Dilutions should be done with a dilution buffer containing a carrier RNA instead of pure H20 and should always be diluted fresh from an aliquoted plasmid stock. It is recommended to prepare a standard curve once and then use it as an external standard for consecutive experiments to minimize variations which might arise from slightly different standard curves.
- For the quantification of total HBV DNA or HDV RNA we use primers and probes available from the TaqMan® Gene Expression Assay System (Life Technologies) with the assay ID Pa03453406_s1 and the standard Taqman PCR program from the Viaa7 or HDV-specific primer and probe (s. methods) and the standard one step protocol, which includes an initial reverse transcription step. The HDV template is denaturized for 10min at 95C° to destroy RNA self-complementary secondary structures and is kept immediately on ice to avoid renaturation of the RNA prior pcr setup.
- PCR setup HBV:
| ABI Fast advance Master |
5 µl |
| Primer and probe mix (final concentration 0.25 µM) |
0.5 µl |
| Sample DNA |
4.5 µl |
| add H2O to a final volume of 10µl |
0 µl |
| ABI fast virus 1-step Master |
2,5 µl |
| Primer mix for+rev (final concentration 0.5 µM) |
1 µl |
| probe (final concentration 0.4 µM) |
0.5 µl |
| Sample RNA |
5 µl |
| add H2O to a final volume of 10µl |
1 µl |
- For calculation of the titer (copies/ml) the copy number result from PCR must be multiplied: Here, 5µl sera were cleaned up, eluted in 25µl H2O and from this 4.5µl (for HBV) or 5µl (for HDV) were used as PCR template, therefore copy number should be multiplied by 1111 for HBV and 1000 for HDV.
Note: If the exact viral nucleotide sequence of the viral inoculum is not known (i.e. unknown patient derived sera) or the development of viral mutations is expected it will be necessary to ensure the efficient primer binding by sequencing of the input virus since even single mismatches can result in false negative or lower RT-PCR quantifications. To minimize variations of the viral copy numbers derived by different clean-ups (i.e. room temperature fluctuations in the lab) and measurements, all samples from an experiment should be processed in parallel whenever it is possible.
Kinetics of HBV and HBV/HDV during the spreading phase in humanized mice
- The spreading of the virus can be monitored by weekly blood withdrawal and the volume of blood should be kept small (≤50µl). The full blood can be taken by a capillary from the mouse eye or from the tail vein.
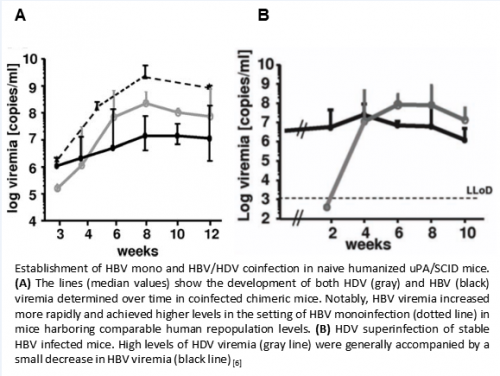
- Depicted below is an example of titer kinetics with humanized mice (30%-50% of total hepatocytes are human in the mouse liver) either i.p. mono-infected with 1E7 copies HBV GT D, coinfected with 1E7 copies of both HBV and HDV (GT1) or stable HBV infected mice were superinfected with HDV (Gt1) (Figure).
- Viremia rises in the following weeks and 3 weeks after infection it ranges, in general, from 1E5 to 5E6 Mio HBV genome equivalents /ml. At 8 weeks, viremia ranges between 1E7 to 1E9 HBV copies/ml and in most cases it becomes stable in HBV mono infection after 12 weeks.
- In mice simultaneously inoculated with HBV and HDV both viruses can be detected in mouse serum samples after 3 weeks of infection, although the development of HBV viremia is frequently slightly slower and/or remains lower as in HBV mono-infection.
- In HDV superinfected mice which have already a stable HBV infection, the HBV titer is slightly reduced while HDV viremia is rising.
Factors possibly affecting the viral kinetics
- the human repopulation level of the mice
- amount of viral inoculum
- viral genotype and mutation pattern
- To some extent also the route of infection (i.v. or i.o. injection gives faster viral spreading compared to i.p. injection, likely due to a higher number of virions reaching the liver with the blood stream and hence cells that are initially infected directly after inoculation
Note: In general, the higher the repopulation level of the mouse, the faster a stable titer is achieved. In lower repopulated mice, the viral spread takes much longer until a stable titer is developed.