
In this episode, Bright and Anousha chat with Dr. John Tavis, a professor, and scientist at St. Louis University School of Medicine, about current drug candidates for #hepatitisB treatment and updates about the cure.

In this episode, Bright and Anousha chat with Dr. John Tavis, a professor, and scientist at St. Louis University School of Medicine, about current drug candidates for #hepatitisB treatment and updates about the cure.

On behalf of the Canadian Hepatitis B Network (CanHepB) and the Canadian Association for the Study of the Liver (CASL), we are pleased to invite you to join us at the Inaugural Progress toward Hepatitis B Elimination Meeting in Canada, September 29th-October 1st 2023.
This meeting will be a unique opportunity to exchange ideas, promote collaboration and foster knowledge translation among Canadian clinicians, laboratory providers, public health researchers, policymakers, and academic & community-based organizations with an interest in hepatitis B virus (HBV) and/or hepatitis Delta virus (HDV) coinfection. Moreover, this inaugural meeting will support advocacy efforts in Canada to increase awareness, reduce stigma, and health care disparities, and improve the care of persons living with hepatitis B or hepatitis delta coinfection.
Despite the availability of an effective vaccine for over 4 decades, hepatitis B continues to impact over 300 million people globally and at least 250,000 Canadians. Hepatitis B is the major global cause of end-stage liver disease and liver cancer. Every 30 seconds someone dies from hepatitis B. Current oral antiviral therapy reduces liver disease risk but cannot cure hepatitis B, thus requiring lifelong and often expensive treatment.
There is a global race to find a cure for hepatitis B. While we await a cure, Canada needs to develop a nationally coordinated strategy to prevent hepatitis B, identify affected persons, and improve access to care and life-saving treatment.
For the first time, our program will bring together national and global leaders in hepatitis B to discuss how Canada can address this major public health threat.
Sincerely,

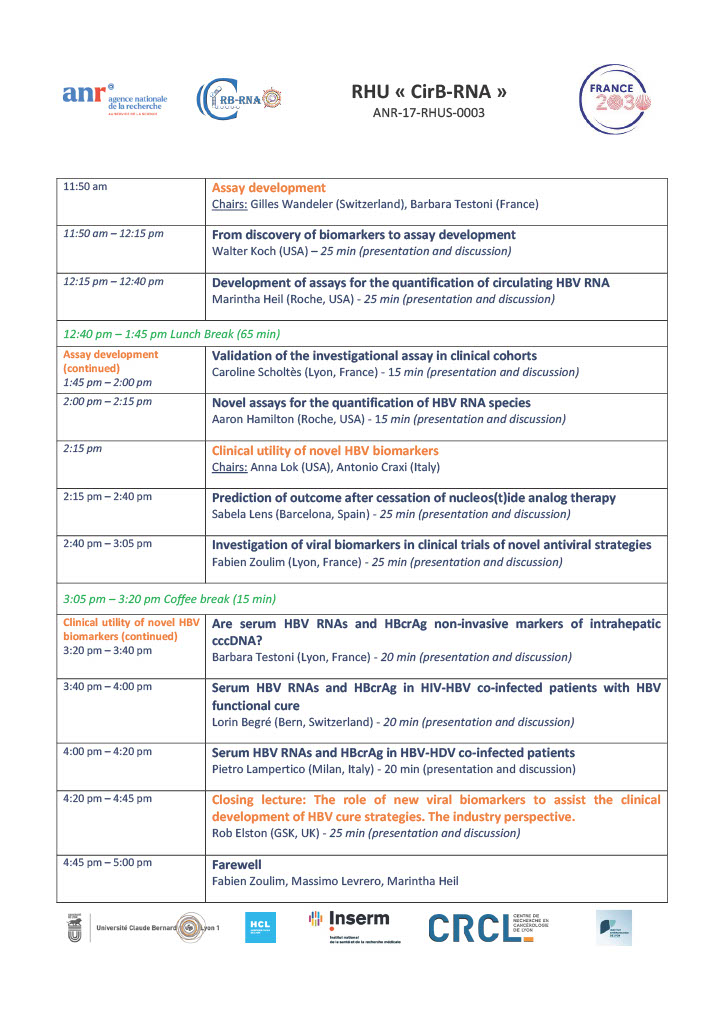
We are delighted to announce that ICE-HBV, as a long-standing partner, will be participating in the International Workshop on viral biomarkers organized by Pr. Fabien Zoulim as part of the CirB-RNA RHU, funded by the ANRS (French national public research agency). The event is scheduled to take place on Thursday, September 7, 2023, at the Institut Lumière, located at 15 Rue du Premier Film, 69008 Lyon, France. The deadline for registration is September 1st, 2023.
Seize this opportunity to engage, learn, and network with leading experts in the field.
https://www.cirb-rna.fr/workshop-september-2023-lyon-france.html

Join the Coalition for Global Hepatitis Elimination and ICE-HBV for a compelling webinar on July 20th from 10:00 am to 11:30 am ET. This webinar aims to provide a comprehensive overview of the current global status of hepatitis D (HDV) elimination. With the availability of new therapeutic options, the webinar will review the burden of HDV, testing challenges, advancements in therapy, and recent insights gained from Bulevirtide. Don’t miss this opportunity to stay informed.
https://taskforce-org.zoom.us/webinar/register/WN_0mIb6otsTYOl3EerjDyHYQ#/registration
https://www.globalhep.org/webinars/global-hdv-elimination-challenges-and-opportunities

Dear Scientific Community,
The William Mason Memorial Symposium will celebrate Bill’s tremendous contributions and scientific legacy. We are honored and humbled to remember him as a pure, scholarly scientist and a kind, generous person.
This event will feature three sessions of oral presentations from Bill’s colleagues and friends on Monday, June 12, beginning at 10 a.m. EDT.
Click on the button below to register.
We will also be collecting well wishes to include in the program, please click the button below to upload. These must be received by Monday, June 5.
Endorsed by:







A greater understanding of the hepatitis B virus (HBV)
replication cycle has led to novel antiviral targets, interfering
with nucleocapsid assembly and disassembly.
In its role as a viral structural protein, the core protein
(HBc) forms a capsid of 120 HBc dimers that packages
the viral genome. Assembly agonists favour formation
of aberrant capsids or morphologically normal capsids
devoid of genetic material1. A number of such ‘capsid
assembly modulators’ (CAMs) are in clinical development.
However, a lack of consistent nomenclature
for these drugs has generated confusion. Compounds
targeting HBc have been described as CAMs, core
protein allosteric modulators (CpAMs), core or capsid
inhibitors, core-targeting agents, and as subclasses
(with inconsistent use on what is class 1 or 2, CAM-A
(aberrant), CAM-E (empty) and CAM-N (normal))2.
As HBc-targeting compounds are undergoing clinical
trials, a consistent classification would improve clarity.
To address the need for a convention that appropriately
categorizes these molecules and provides clear,
precise language to document their development, the
HBV Forum and ICE-HBV jointly convened a working
group (Supplementary Box 1) to develop a standardized
nomenclature that accommodates mechanisms of action
(MOAs) whilst being simple, intuitive and accessible.
The next video of our #HBVCureFAQs series is out!! See the video where Dr Katharina Bartsch & Laura Kaltenberg of CTC North, collaborating in TherVacB, answer “How safe are #clinicaltrials & what should patients expect?” here.
The symposium, chaired by Mala Maini and Antonio Bertoletti, debated challenges surrounding immunotherapies for HBV and discussed ways forward.
The videos from this event are on ICE-HBV YouTube channel:
Welcome to the 3rd HBV Community Workshop co-organised by The Hepatitis B Foundation and ICE-HBV in Paris on 22 September 2022.
Click here to watch the video.
See the call to action from the community forum here.