Introduction
- Although a plethora of knowledge on the molecular life cycle of Hepatitis B Virus has been gained by utilizing classical methods, they can only inform on the average level of the tested molecule without subcellular or histological context1.
- Here we describe a modified FISH protocol that allows direct visualization of HBV minus and plus strand DNA in cell culture models (e.g. HepAD38, HepG2-NTCP)2. It can be coupled with immunofluorescence staining of viral or host proteins or any other fluorescent tagging system which could illuminate numerous aspects of virus-host interactions.
- Our protocol is modified from the ViewRNA ISH cell assay (Thermo Fisher). It is based on a branched DNA signal amplification scheme in which target sequences are hybridized to a series of synthetic oligonucletodies (probe set, preamplifier, amplifier and label probe) and realizes high sensitivity and low background. We found that with some modifications, it also works well for HBV DNA.
Materials and Reagents
- REAGENTS
- PBS (Cell culture grade)
- 3.7% formaldehyde (Dilute the 37% formaldehyde stock solution in PBS to obtain a final concentration of 3.7% formaldehyde.)
- Deionized water (ddH2O)
- 100% ethanol; 70% ethanol (Add seven volumes 100% ethanol to three volumes deionized water); 50% ethanol (Add one volume 100% ethanol to one volume deionized water.)
- Ribonuclease A, RNase A (Code No.2158, TaKaRa, Japan)
- Ribonuclease H, RNase H (Cat. EN0202, Thermo Fisher, Waltham, MA, USA)
- Rubber cement (Cat. BEACON ADHESIVES, Mount Vernon, NY, USA)
- QuantiGene ViewRNA ISH cell assay kit (Cat No. QVC-0001, Thermo Fisher, Waltham, MA, USA), contains Protease QS, Probe Set Diluent QF, Amplifier Diluent QF, Label Probe Diluent QF, PreAmplifier Mix, Amplifier Mix, Label Probe Mix, Wash Buffer Component 1 (Wash Comp 1), Wash Buffer Component 2 (Wash Comp 2), 10 X DAPI
- QuantiGene ViewRNA Probe sets:
- Probeset 2: VF6-16995 (TYPE 6) (Thermo Fisher) is complementary to the minus strand of the HBV sequence (target region: nt 2957-837, reference: AF100309)2;
- Probeset 3: VF1- 20344 (TYPE 1) is complementary to the 1kb gap region of the plus strand (nt 500-1590, reference: AF100309) 2.
- Hoechst 33342 (Cat. H3570, Molecular Probes, Eugene, Oregon, USA)
- Fluorescence Mounting Medium (Code S3023, DAKO, Denmark)
- Clear Nail Polish
- Cell culture medium: DMEM medium supplemented with 10 % fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin
- Replication medium: culture medium plus 2.5% DMSO
- Concentrated HBV: Supernatants of HepAD38 cells (dox−) were collected and concentrated 100-fold by ultrafiltration (Amicon Ultra, 100 kDa; Millipore, Burlington, MA, USA)
- EQUIPMENTS
- Nunc Lab-Tek II four/eight-well Chamber Slide (Cat No. 154526 (four-well) or Cat No. 154534 (eight-well), Thermo Fisher, Waltham, MA, USA). Before cell seeding, chamber slides are coated with collagen (Rat tail collagen, Gibco A10483-01 , diluted 20 fold in PBS ) for 20 minutes and washed twice with PBS.
- Microcentrifuge
- Water bath (set to 37 °C and 40° C)
- High-Temperature Drying Oven (set to 75 ℃)
- Thermobrite hybridizer (If no hybridizer is available, one can also use stainless steel cassettes to hold slides and let it float on a water bath. The cassette should be humified using pre-wetted tissue paper.)
- 24×50 mm2 coverslips
- Forceps
- Fume hood
- Fluorescent microscope.
Experimental Procedures
Sample preparation-
Cell culture and HBV infection.
1.1 Tetracycline-inducible (tet-off) HepAD38 were seeded on collagen-coated four-well chamber slides and maintained in culture medium for 48 - 72 h.
1.2 HepG2-NTCP cells were cultured in collagen-coated four-well chamber slides, and at approximately 100% confluence, concentrated supernatants of HepAD38 cells were mixed with culture medium containing 4% PEG8000 and inoculated onto cells for 6 - 8 h. The cells were subsequently washed three times with PBS and maintained in replication medium for several days based on the aim of experiment.
-
Fix the cells in 3.7% formaldehyde solution and dehydration.
2.1 In a fume hood, prepare 6 ml of fresh 3.7% formaldehyde solution by diluting 500 ul of a 37% stock formaldehyde with 5.5 ml of PBS and then mixing well.
▲Caution: Formaldehyde is a poison and irritant. Avoid contact with skin and mucous membranes.
2.2 Gently aspirate off culture medium and rinse cells three times, each time with 300 ul/well of PBS.
- Critical: Before fixation, cell attachment may be weak. Aspiration and dispensing of contents from and into the chamber should be performed slowly and gently with a 1 mL pipette.
Aspirate from the corners and dispense against the chamber wall.
2.3 Aspirate off the final PBS wash and add 300 μL/well of freshly prepared 3.7% formaldehyde solution. Cover chamber slide with lid and incubate at RT for 10 minutes.
2.4 Aspirate off the formaldehyde solution and gently rinse the cells three times, each with 300 ul/well of PBS.
2.5 Aspirate off the PBS and replace it with 300 μL/well of 50% ethanol. Incubate for 5 minutes at RT.
2.6 Aspirate off the 50% ethanol and replace with 300 μL/well of 100% fresh ethanol. Seal the chamber slides with parafilm and store the dehydrated cells in 100% ethanol at –20 °C until useda.
If doing the experiment right away, aspirate off the 50% ethanol and replace with 300 μL/well of 70% ethanol at 4 °C for at least 1 hour and proceed on 3.1.B
NOTE:
- Dehydrated cells can be stored under these conditions for up to one month and must be rehydrated before being used in the in situ assay.
FISH assay procedure
-
Cell rehydration.
3.1 A. Aspirate off the 100% ethanol (corresponding to 2.6 A) and replace with 300 μL/well of 70% ethanol. Incubate for 5 minutes at RT.
B. Aspirate off the 70% ethanol (corresponding to 2.6 B) and replace with 300 μL/well of 50% ethanol. Incubate for 5 minutes at RT.
C. Aspirate off the 50% ethanol and replace with 300 μL/well of PBS. Incubate for 10 minutes at RT.
3.2 Aspirate off the PBS and add 300 μL/well of a 3.7% formaldehyde solution. Incubate for 10 minutes at RT.
3.3 Aspirate off the formaldehyde solution and gently rinse the cells three times, each with 300 μl/well of PBS for 5 minutes at RT.
-
Protease digestiona.
4.1 Prepare 1.2 ml diluted working Protease Solution (1:3000) in PBS by adding 0.4 ul Protease QSb to 1199.6 μl PBS. Vortex briefly to mix.
4.2 Aspirate off the PBS and added 300 μL/well of working protease solution. Cover chamber slides with lid and incubate for 10 minutes at 37 °C in a water bath.
4.3 Aspirate off the working protease solution from the chamber and rinse the cells three times, each with 300 μl /well of PBS.
4.4 Aspirate off PBS and add 300 μL/well of a 3.7% formaldehyde solution. Incubate for 5 minutes at RT.
4.5 Aspirate off the formaldehyde solution and gently rinse the cells three times, each with 300 μL/well of PBS.
NOTES:
- The Protease digestion step partially degrades capsid and polymerase that wrap HBV DNA thus increasing target accessibility. However the exact concentration of protease should be empirically determined because excessive digestion can cause complete release of nucleic acids and even cell morphology damage and cell loss.
- Protease QS is provided in the kit. Thaw and place Protease QS on ice until use.
-
Digest with RNase A/Ha.
5.1 Prepare 1.2 ml diluted working RNase A/H solution by adding 2.4 μl RNase A 1:500 and 2.4 μL RNase H 1:500 to 120 μL 10X RNaseH Reaction buffer and 1075.2 μl deionized water.
5.2 Aspirate off the PBS and added 300 μL/well of working RNase A/H solution. Incubate the slides in a cassette for 1 hour at 37 °C on the water bath.
5.3 Aspirate off the working RNase A/H solution and gently rinse the cells three times, each with 300 μL/well of PBS for 5 minutes at RT.
5.4 Aspirate off the PBS and added 300 μL/well of 3.7% formaldehyde solution. Incubate for 5 minutes at RT.
5.5 Aspirate off the formaldehyde solution and gently rinse the cells three times, each with 300 μL/well of PBS for 5 minutes at RT.
NOTES:- a. RNase A is an endonuclease that specifically degrades single-stranded RNA.
RNase H is an endonuclease, it can decompose the RNA strand in the RNA/DNA hybrids.
Our previous results suggested that HBV RNA could exist in the form of RNA/DNA hybrid in core particles1,3. The combined use of RNase A/H digestion can increase signal and improve assay specificity. First, probe set 3 targets the plus strand of HBV DNA but can also bind to HBV RNA. RNase digestion eliminate residual HBV RNA in the replication complex and capsid-free HBV mRNA. Second, RNaseH digestion eliminate the HBV RNA that binds to minus strand DNA hence increasing the hybridization efficiency of probe set 2.
6. Hybridization with Probe.
6.1 Prepare 150 μl working Probe Set Solution by diluting 1.5 μl of each Probe Set (1:100) in 148.5 μl pre-warmed Probe Set Diluent QFa. Gently mix the solution and shortly centrifuge 10-20 seconds. 150 µl for every slide?
▲Caution: Probe Set Diluent QF contains formamide, a teratogen, irritant and possible carcinogen. Avoid contact with skin and mucous membranes.
6.2 Remove the slides from the chamber and gently decant the remaining PBS, then put the slide edge on a clean, dry paper towel for 1-2 seconds. Add 150 μl/slide of the working Probe Set Solution to the slide, subsequently cover with a 24×50 mm2 coverslip and seal edges with rubber cement.
- Critical: The chamber on the slide may not be easily removed. Handle with care to avoid the slide broken.
6.3 Preheat the cassette at 75 °C in the dry incubator for 2 minutes.
6.4 Then incubate at 40 °C for 3 hours in the water bath or the Thermobrite hybridizer.
NOTE:
- Pre-warm Probe Set Diluent QF to 40 °C in a water bath for 10 minutes to dissolve possible precipitates.
7. Hybridize with Pre-Amplifier
7.1 Prepare 15 ml Wash Buffer by adding 45 μl Wash Comp1 and 75 μl Wash Comp2a to 14.88 ml deionized water and then mixing well.
7.2 Prepare 150 μl working Pre-Amplifier Mix Solution by diluting 0.75 μl Pre-Amplifier Mix 1:200 in 149.25 μl pre-warmed Amplifier Diluent QF. Gently mix the solution and transient centrifuge for 10-20 seconds.
▲Caution: Amplifier Diluent QF contains formamide, a teratogen, irritant and possible carcinogen. Avoid contact with skin and mucous membranes.
7.3 Remove rubber cement and coverslips from the slide, then put the slide edge on a clean, dry paper towel for 1–2 seconds. Wash slides three times, each with 1 mL/slide of Wash Buffer. Soak sample for 5 minutes during each wash.
- Critical: During all subsequent washing steps, the slides should be washed fully with wash buffer to eliminate non-specific binding, but do not soak samples in Wash Buffer longer than 30 minutes. Are all the washing steps at room temperature?
7.4 Gently remove the Wash Buffer, put the slide edge on a clean, dry paper towel for 1–2 seconds. Add 150 μl/slide of the working PreAmplifier Mix Solution to the slide, then cover with a 24×50 mm2 coverslip.
7.5 Incubate at 40 °C for 35 minutes in the water bath.
NOTES:
- Wash Comp 1 and Wash Comp 2 are provided in the kit, they should be free of precipitates before making a working wash buffer.
8. Hybridize with Amplifier
8.1 Prepare 150 μl working Amplifier Mix Solution by diluting 6 μl Amplifier Mix 1:25 in 144 μl pre-warmed Amplifier Diluent QF. Gently mix the solution and transient centrifuge for 10-20 seconds.
▲Caution: Amplifier Diluent QF contains formamide, a teratogen, irritant and possible carcinogen. Avoid contact with skin and mucous membranes.
8.2 Remove coverslips from the slide, then put the slide edge on a clean, dry paper towel for 1–2 seconds. Wash slides three times, each with 1 mL/slide of Wash Buffer. Soak sample for 5 minutes during each wash.
8.3 Gently aspirate the Wash Buffer, put the slide edge on a clean, dry paper towel for 1–2 seconds. Add 150 μl /slide of the working Amplifier Mix Solution to the slide, then covered with a 24×50 mm2 Cover Slip.
8.4 Incubate at 40 °C for 35 minutes in the water bath.
9. Hybridize with Label Probe
- Critical: Protect samples from light during all subsequent steps.
9.1 Prepare 150 μl working Label Probe Mix Solution by diluting 6 μl Label Probe Mix 1:25 in 144 μl pre-warmed Label Probe Diluent QF. Gently mix the solution and shortly centrifuge for 10-20 seconds. Protect from direct light exposure.
9.2 Remove coverslips from the slide, then put the slide edge on a clean, dry paper towel for 1–2 seconds. Wash slides three times, each with 1 mL/slide of Wash Buffer. Soak sample for 5 minutes during each wash.
9.3 Gently aspirate the Wash Buffer, put the slide edge on a clean, dry paper towel for 1–2 seconds. Add 150 μl /slide of the working Label Probe Mix Solution to the slide, then cover with a 24×50 mm2 coverslip.
9.4 Incubate at 40 °C for 25 minutes in the water bath.
10. Nuclear counterstaining
10.1 Prepare 500 μl working Hoechst Solution by diluting the 0.5 μl Hoechst 1:100 in 999.5 μl PBS. Gently mix the solution. Protect from light.
Note: Nuclear staining can also be done with DAPI. Dilute the 100 X DAPI (provided in the ViewRNA ISH cell assay kit) 1:100 in nuclease free water to obtain a working DAPI solution and perform as follows.
▲Caution: DAPI is a possible mutagen. Avoid contact with skin and mucous membranes.
10.2 Remove coverslips from the slide, then put the slide edge on a clean, dry paper towel for
1–2 seconds. Wash slides three times, each with 1 mL/slide of Wash Buffer. Soak sample for 5 minutes during each wash.
10.3 Gently aspirate the Wash Buffer, put the slide edge on a clean, dry paper towel for 1–2 seconds. Add 500 μl/slide of the working Hoechst Solution to the slide and incubate for 3 minutes at RT.
10.4 Decant working Hoechst Solution from the slide. Wash slides three times with 1 mL/slide of PBS. Soak sample for 5 minutes during each wash.
10.5 Aspirate PBS and add two drops of fluorescent mounting medium onto the slide, and finally cover with a coverslip to preserve a better fluorescent signal.
10.6 Use a clean paper towel to soak up the excess mounting medium on the sides of the slide when necessary, and seal the slide edges with nail polish.
10.7 Allow the nail polish to dry for at least 15 minutes.
11. Imaging
A. Samples may be viewed under a fluorescent microscope immediately.
B. The slide should be stored at 2–8°C protected from light. Fluorescent signals will be stable for up to one week when stored properly.
C. For viewing signals, use appropriate filter sets. Here we can use filter set Cy3 (550 nm) to detect Probe Set type 1a or use filter set Cy5 (650 nm) to detect Probe Set type 6b.
NOTES:
a. Signal of LP1-550 is visible to unaided eyes under epi-fluorescent microscope, and appear as red dots.
b. Signal of LP6-650 is invisible to unaided eyes under epi-fluorescent microscope and has to be captured by a digital camera.
c. Due to the relative weak signal of FISH, a digital camera with high quantum efficiency is desirable. In addition, high sensitivity mode (e.g. HyD mode in Leica SP8) must be used when using a confocal microscope. The signal is also more prone to be bleached due to the high energy of confocal laser beam. We have also imaged the FISH results using STORM (Type 6 probe only) and STED with some success. Generally, these methods generate higher resolution images but the procedure is much more complex.
d. If one wishes to see colocalization between nucleic acid and protein, a routine immunofluorescence protocol can be performed immediately after 10.2 with no need for fixation or permeabilization step.
-
Typical results
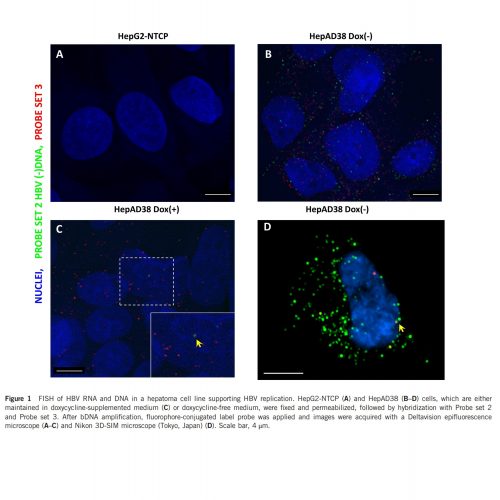
Typical imaging results were shown below2. Signal generated by probe set 2 (green) indicates all viral DNA (including core particle DNA and deproteinated DNA), signal generated by probe set 3 (red) indicates deproteinated rcDNA and some core particle DNA in the cytoplasm. Our previous results showed that the rcDNA in HepAD38 has longer plus strand which resulted in a diminished selectivity of probe set 3 toward intranuclear rcDNA and cccDNA. The yellow arrow indicates intranuclear HBV DNA that is detected by both probes. Further differentiation between episomal DNA and integrated HBV DNA is beyond the capability of the assay.
More images are also available at http://www.hepb-atlas.com/home/projects/detail/12
References
- Zhang X, Lu W, Zheng Y, Wang W, Bai L, Chen L, Feng Y, Zhang Z, Yuan Z (2016) In situ analysis of intrahepatic virological events in chronic hepatitis B virus infection. J Clin Invest 126:1079-1092.
- Zhang X, Yue L, Zhang Z, Yuan Z (2017) Establishment of a fluorescent in situ hybridization assay for imaging hepatitis B virus nucleic acids in cell culture models. Emerg Microbes Infect 6:e98.
- Bai L, Zhang X, Kozlowski M, Li W, Wu M, Liu J, Chen L, Zhang J, Huang Y, Yuan Z. Extracellular Hepatitis B Virus RNAs Are Heterogeneous in Length and Circulate as Capsid-Antibody Complexes in Addition to Virions in Chronic Hepatitis B Patients. J Virol. 2018;92(24). pii: e00798-18.