Glossary Terms List
| ARV | Antiretroviral |
| ART | Antiretroviral Therapy |
| BE | Bioequivalence |
| CHAI | Clinton Health Access Initiative Inc. |
| CO | Country Office |
| CPT | Carriage Paid To |
| CRP | Collaborative Registration Procedure |
| CT / NG | Chlamydia Trachomatis / Neisseria Gonorrhea |
| CY | Calendar Year |
| DAA | Direct-Acting Antivirals |
| DAP | Delivery At Place |
| DCV | Daclatasvir |
| EID | Early Infant Diagnosis |
| ERP | Expert Review Panel |
| FDC | Fixed-Dose Combination |
| FDF | Finished Dosage Form |
| FOB | Freight On Board |
| FPP | Finished Pharmaceutical Product |
| GFATM | The Global Fund to Fight AIDS, Tuberculosis and Malaria |
| G/P | Glecaprevir / Pibrentasvir (Fixed-Dose Combination) |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C Virus |
| HIV | Human Immunodeficiency Virus |
| IA | Immunoassay |
| LDV | Ledispasvir |
| LMIC | Low-and Middle-Income Country |
| MoH | Ministry of Health |
| MPP | Medicines Patent Pool |
| MTB | Mycobacterium Tuberculosis |
| NAT | Nucleic Acid Test |
| PAHO | Pan American Health Organization |
| PCR | Polymerase Chain Reaction |
| PPM | Pooled Procurement Mechanism |
| QA | Quality Assured |
| RBV | Ribavirin |
| RDT | Rapid Diagnostic Test |
| RNA | Ribonucleic Acid |
| SOF | Sofosbuvir |
| SOF / DCV | Sofosbuvir / Daclatasvir (Fixed-Dose Combination) |
| SOF + DCV | Individual Sofosbuvir and Daclatasvir Combined |
| SOF / LDV | Sofosbuvir / Ledispasvir (Fixed-Dose Combination) |
| SOF / VEL | Sofosbuvir / Velpatasvir (Fixed-Dose Combination) |
| SRA | Stringent Regulatory Authority |
| SVR12 | Sustained Virologic Response at week 12 |
| VEL | Velpatasvir |
| VL | Viral Load |
| WHO PQ’d | World Health Organization Prequalified |
Hepatitis Commodities Access in Resource-Limited Settings
With the below mapping of hepatitis procurement processes for prevention tools, diagnostics, monitoring and treatment, the aim is to increase awareness and information on cost and procurement within the hepatitis community, in order to increase access to viral hepatitis medicines and technologies in low-and-middle income countries, including HBV birth-dose vaccine, HBV treatment and HCV cure uptake.
The mapping shows how to access hepatitis commodities at the best available price in your country. Prices are given as examples and are subject to change. The prices indicated below usually do not include freight, taxes and distribution costs.
The mapping was built in partnership with ICE-HBV Stakeholders and in particular the Medicines Patent Pool and the Clinton Health Access Initiative (Hepatitis C Report).
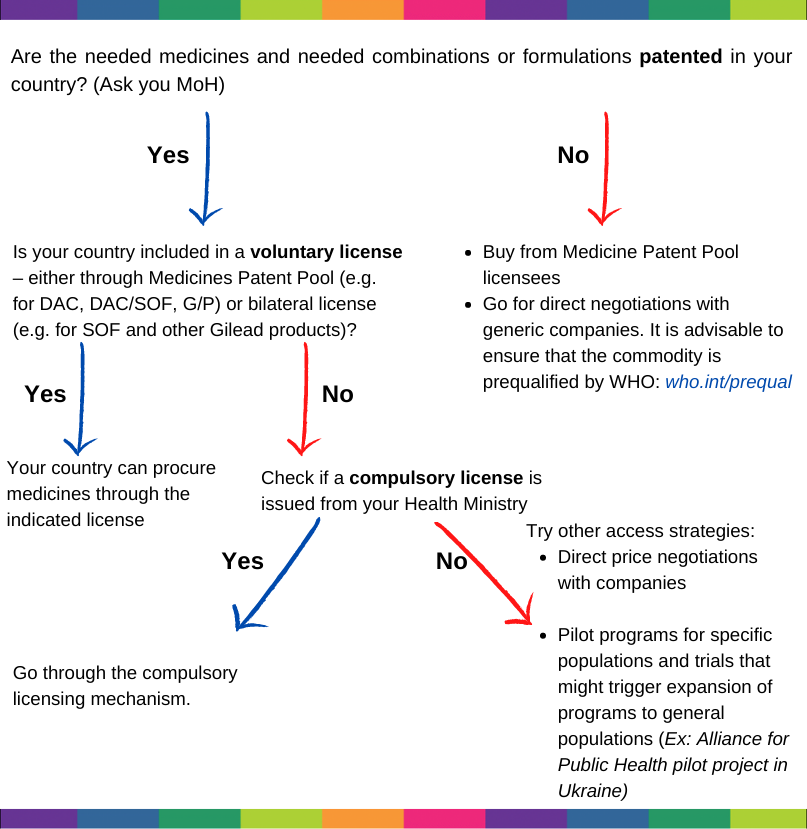
When looking into strategies to access viral hepatitis medicines in your country, the following questions are key starting points:
- First, check the WHO Global Health Sector Strategy on Viral Hepatitis.
- Is there a national viral hepatitis program and does it include prevention, testing, treatment and care for HBV and HCV alike?
- Is there enough budget for implementing the national program and/or what are the options for external funding?
- What medicines for treating viral hepatitis are on the national Essential Medicine List (EML)? Is the list in line with WHO EML? Are the medicines/diagnostics pre-qualified by WHO or approved by other regulatory bodies (FDA, EMA, etc)
- Are procurements happening within the frame of the national program? Are medicines procured directly by your Ministry of Health or through procurement agencies?
- How many people are on treatment in your country? How many people pay out of pocket?
Procurement cycle
- Plan your procurement ahead so you have a better position for negotiation and potentially reduce the end price per patient.
- Make sure the prices you negotiate include the country mark-ups (taxes, margins, etc.)
- Follow the cycle below:
Who to contact for quality insured pooled procurement:
Depending on their country, buyers can generally obtain better prices by using pooled procurement systems and patent pools licences such as these:
Medicines Patent Pool (MPP) & MedsPaL Database of Licenses
Main Database: medspal.org
MPP does not sell commodities, they negotiate voluntary licences for generic companies to produce quality-insured medicines. The list of available licences can be found on the MedsPal database. If a country is not covered by a MPP licence. Countries can approach MPP with a request to include a country in a licence using the contact below.
Contact: Mila Maistat
E-mail: lmaistat@medicinespatentpool.org
IDA Foundation
Headquarters (Holland): idafoundation.org
Phone: +31 20 4033051
Regional Offices: idafoundation.org/en/who-we-are/offices
The Global Fund to Fight AIDS, Tuberculosis and Malaria
For Medicines, contact Uranchimeg Badarch at badarch@theglobalfund.org
For Diagnostics, contact Azizkhon Jafarov at jafarov@theglobalfund.org
UNDP
Contact: Zafar Yuldashev
E-Mail: zafar.yuldashev@undp.org
UNICEF Procurement
- Contact: Aadrian Sullivan
- Email: asullivan@unicef.org
- Website: https://www.unicef.org/supply/procurement-services-resources-and-guidelines
Commodities Pricing Database (June 2021)
HBV Vaccines
HBV Vaccines
WHO Guidelines Recommendations:
- Infants: First dose within 24 hours of birth. Followed by 2 or 3 doses to complete primary series.
- No booster dose is required/supported by evidence.
- Adult catch-up vaccination recommended.
WHO EML Listed?
- Yes
Possible Quality Insured Pooled Procurement Systems:
- UNICEF:
- Children and adults: $0.24 USD to $0.30 USD/dose
- Adult single dose: $0.70 USD/dose
HBV Birth-Dose Vaccine
WHO Guidelines Recommendations:
- Should be given within 24 hours of birth.
WHO EML Listed?
- Yes
Possible Quality Insured Pooled Procurement Systems:
- UNICEF: $0.24 USD to $0.60 USD/dose
HBV Testing & Monitoring
Rapid Diagnostic Tests
WHO Guidelines Recommendations
- Recommended for detection of HBsAg and delivered at point of care to improve access and linkage to care and treatment.
Possible Quality Insured Pooled Procurement Systems:
- Global Fund: $0.90/test
HBV Viral Load Test
WHO Guidelines Recommendations:
- Global Fund: Cepheid Xpert® HCV Viral Load – USD 16.55
HBV Antivirals
HBV Antivirals
WHO Guidelines Recommendations:
- WHO (2015) Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection.
Entecavir
WHO Guidelines Recommendations:
- Preferred use for first- and second-line treatment.
WHO EML Listed?
- Yes
MPP Licensed?
Tenofovir Disoproxil TDF
WHO Guidelines Recommendations:
- Recommended
WHO EML Listed?
- Yes
MPP Licensed?
Possible Quality Insured Pooled Procurement Systems:
- Global Fund: $28.80/year
- UNDP: $23/year
- IDA Foundation: $57/year
- UNICEF: $31/year
Tenofovir Alafenamide TAF
MPP Licensed?
Possible Quality Insured Pooled Procurement Systems:
HCV Testing & Monitoring
Rapid Diagnostic Tests
WHO Guidelines Recommendations:
- Recommended for detection of HCV antibody at point of care to improve access and linkage to care and treatment.
Possible Quality Insured Pooled Procurement Systems:
- Global Fund: $1.10/test
HCV Viral Load
WHO Guidelines Recommendations:
- Quantitative NAT is recommended.
Possible Quality Insured Pooled Procurement Systems:
HCV Cures
Daclatasvir
WHO Guidelines Recommendations:
- Recommended use in combination with Sofobuvir in people aged ≥18 as combination is pan genotypic.
- Recommended not in combination with other drugs in people aged 12-17 with susceptible genotypes.
WHO EML Listed?
- Yes
MPP Licensed?
Possible Quality Insured Pooled Procurement Systems:
- Global Fund: $79
Sofosbuvir
WHO Guidelines Recommendations:
- Recommended use in combination with Daclatasvir in people aged ≥18 as combination is pan genotypic.
- Recommended not in combination with other drugs in people aged 12-17 with susceptible genotypes.
WHO EML Listed?
- Yes
MPP Licensed?
Possible Quality Insured Pooled Procurement Systems:
- Global Fund: $79
Sofosbuvir + Daclatasvir (12 weeks)
WHO Guidelines Recommendations:
- Recommended in people aged ≥18 years.
WHO EML Listed?
- Yes, but as separate drugs (not as a single drug).
MPP Licensed?
Possible Quality Insured Pooled Procurement Systems:
- Global Fund: $94
- UNDP: $89
- IDA Foundation: $85
Sofosbuvir + Velpatasvir
WHO Guidelines Recommendations:
- Recommended in people aged ≥18 years.
WHO EML Listed?
- Yes
MPP Licensed?
Possible Quality Insured Pooled Procurement Systems:
- UNDP: $270
Sofosbuvir + Ledipasvir
WHO Guidelines Recommendations:
- Recommended +/- ribavirin.
WHO EML Listed?
- Yes
MPP Licensed?
Glecaprevir / Pibrentasvir
WHO Guidelines Recommendations:
- Recommended in people aged ≥18 years.
WHO EML Listed?
- Yes
