Author's Information
Conan Chua, Aman Mehrotra, Dr. Adam Gehring
Toronto Centre for Liver Disease, Toronto, Ontario, Canada
Introduction
During a traditional FluroSpot assay, low HBV Specific T cell frequencies have hindered effective ex vivo analysis. We overcame this obstacle to measure ex vivo T cell responses in CHB patients, by modifying the key variables of cell number and the peptide pulsing method to improve ex vivo detection of HBV-specific T cells.
Materials and Reagents
- 107 frozen PBMCs (per donor; maximum 6 donors per plate)
- 30ml polypropylene tube (one per donor sample)
- 7ml Eppendorf tubes
- 3-color FluoroSpot kit Catalog: hT3002F(ImmunoSpot; 6 donors per plate)
- HBSS medium (Gibco, Ref# 24020-117)
- AIM V medium (Gibco, Ref# 12055-091)
- Knock out serum Replacement (KSR) (Lifetech: 10828028)
- Primocin ( 1ml of 50mg/ml: InvivoGen: Cat: ANT-PM-05)
- Human Blood type-AB serum (VWR, CA45001-062)
- DMSO solution (Sigma, Ref# D8418)
- HBV overlapping peptides (OLP), genotype C (GenScript) (Make Superstock:50mg/ml in 100% DMSO)
- 250ug/ml (50X) OLP pools: (made from superstock)
- 50X DMSO control media: (38% DMSO in AIM V)
- CEF purified peptide library (GenScript):
- DynabeadsTM Human T-activator CD3/28 (Gibco, Ref# 11131D)
- ImmunoSpot S6 Universal Analyzer
- IPFL plate (Merck Millipore, Ref# S53J104I07-MB) (comes with the kit)
- 50mL multi-pipette troughs (Optional)
- Multi-channel pipette (Optional)
- Repeater pipette (Optional)
- Syringes
- 22µm filter (Merck Millipore, Ref# SLGP033RS)
- Vacuum manifold or plate washer
- 70% Ethanol (in PBS)
- Blocking solution (AIM V media with 10% Knockout Serum)
- AIM V with Primocin (add 1ml of Primocin to 500ml bottle of AIM V)
Experimental Procedures
Preparation of OLP peptide pools:
- 313 peptides in total
- Spans 15 amino acids (ie. 15-mers)
- Peptide offset: 5 amino acids apart
- Peptide pools:
- PreCore/Core = 41 peptides
- X = 29 peptides
- Env - 1 = peptides 1 – 181 (37 peptides)
- Env – 2 = peptides 186 - 376 (36 peptides - 3 peptides not synthesized: 241, 246, 251)
- Pol-1 = peptides 1 – 206 (42 peptides)
- Pol-2 = peptides 211 – 416 (42 peptides)
- Pol-3 = peptides 421 – 626 (42 peptides)
- Pol-4 = peptides 631 – 831 (41 peptides)
Preparation of 50X OLP peptide pool
- PreCore/Core 50x
- 41 peptides x 10 μl /peptide = 410 μl of peptides + 590 ml Aim-V + Prim
- X 50x stimulation pool
- 29 peptides x 10 μl /peptide = 290 μl of peptides + 710 ml Aim-V + Prim
- Envelope 50x stimulation pool
- Env-1 37 peptides x 10 μl /peptide = 370 μl of peptides + 630 ml Aim-V + Prim
- Env-2 36 peptides x 10 μl /peptide = 360 μl of peptides + 640 ml Aim-V + Prim
- Polymerase 50x stimulation pool
- Pol-1 42 peptides x 10 μl /peptide = 420 μl of peptides + 580 ml Aim-V + Prim
- Pol-2 42 peptides x 10 μl /peptide = 420 μl of peptides + 580 ml Aim-V + Prim
- Pol-3 42 peptides x 10 μl /peptide = 420 μl of peptides + 580 ml Aim-V + Prim
- Pol-4 41 peptides x 10 μl /peptide = 410 μl of peptides + 590 ml Aim-V + Prim
Ex Vivo 3-color FluoroSpot – Cell prepping (ImmunoSpot)
Materials:
- 107 frozen PBMCs (per donor; maximum 6 donors per plate)
- 30ml polypropylene tube (one per donor sample)
- Eppendorf tubes
- 3-color FluoroSpot kit (ImmunoSpot; 6 donors per plate)
- HBSS medium (Gibco, Ref# 24020-117)
- AIM V medium (Gibco, Ref# 12055-091)
- Knock out serum Replacement (KSR) (Lifetech: 10828028)
- Primocin ( 1ml of 50mg/ml: InvivoGen)
- Human Blood type-AB serum (VWR, CA45001-062)
- HBV overlapping peptides, genotype C (GenScript)
- CEF purified peptide library (GenScript): (USED to monitor treatment effect on unrelated virus-specific T cells)
- DMSO solution (Sigma, Ref# D8418)
- DynabeadsTM Human T-activator CD3/28 (Gibco, Ref# 11131D) (USED as positive/Assay Control)
Day 1: Resting cells (pre-warm media)
- Thaw 107 cells with HBSS medium (as per protocol) in a 30mL polypropylene tube
- Minimum count required for experiment: 6 x 106cells/donor
- Centrifuge at 300xg for 5mins., aspirate
- Resuspend at approximately 4x106 cells/ml with AIM V (+2% human serum)
- Take counting aliquot for pre-rest counts
- fix cell concentration if necessary (3.5-4.5x106 cells/ml are suitable as well)
- Incubate O/N in 37oC incubator @ 5% CO2
- Coat IPFL plates, fridge O/N in 4oC (as per manufacturer’s, see next pages)
Day 2a: Pulsing cells (pre-warm media)
- 16-18h later, resuspend samples and take counting aliquot for post-rest counts
- Aliquot two Eppendorf tubes with 4.5 x 105 cells each (per donor)
- 1 tube for HBV OLP stimulation, another for DMSO vehicle control
- Centrifuge at 300xg for 5mins., aspirate
- Resuspend both tubes with 84µl AIM V (+2% human serum) each
- Pulsing scheme: final volume of 100µl
- HBV OLP sample: add 2µl of 250µg/ml/peptide per OLP pool (8 pools: 16µl)
- HBV OLP final concentration: 5µg/ml/peptide
- DMSO control sample: add 16µl of 19.38% DMSO (in AIM V)
- DMSO final concentration: 3.1% DMSO (equivalent to OLP sample)
- Incubate for 1hr in 37oC incubator @ 5% CO2
- Block IPFL plate simultaneously (as per protocol, see next page)
- Centrifuge both tubes of pulsed cells at 300xg for 5mins., aspirate
- Resuspend cells with 225µl AIM V (no serum) (ie. 2 x 106 cells/ml)
- Aliquot two Eppendorf tubes with 8 x 106 cells each (per donor); centrifuge, aspirate
- Resuspend cells with 450µl AIM V (no serum) (ie. 4 x 106cells/ml)
- Pool respectively with pulsed cells
- Wash tube with another 450µl AIM V (no serum) and pool respectively
- Final volume:25x106 PBMCs in 1.125ml AIM V (ie. 2 x 106cells/ml)
- Aliquot 1.2 x 106cells for CEF controls; centrifuge, aspirate
- Resuspend cells with 588µl AIM V and 12µl 50x CEF (ie. 2 x 106 cells/ml)
- Aliquot 1 x 105cells for CD3/28 controls; centrifuge, aspirate
- Resuspend cells with 400µl AIM V and 0.4µl CD3/28 (ie. 2.5 x 105 cells/ml)
Day 2b: Plating cells
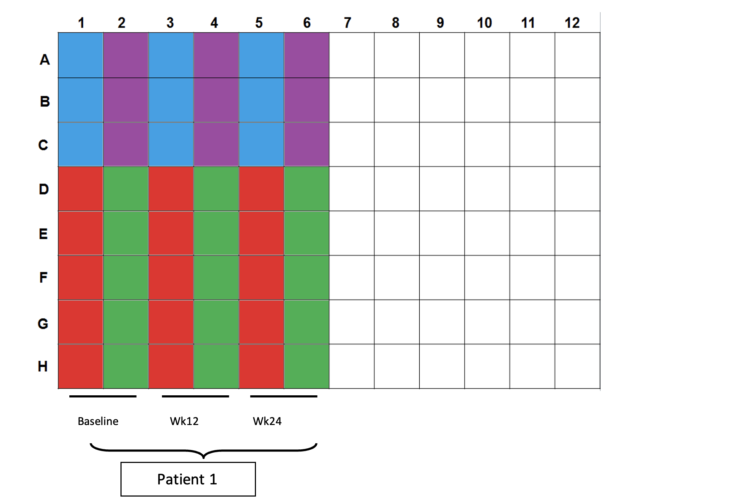
- Color scheme for plating:
- Blue wells: 100µl CD3/28 controls (2.5x104cells per well)
- Purple wells: 200/200/100µl CEF controls (4x105cells/2x105cells per well)
- Red wells: 200µl DMSO controls (4x105cells per well)
- Green wells: 200µl OLP pulsed cells (4x105cells per well)
- Plating totals:
- CD3/28: 5x104 cells in 3 wells
- CEF: 1x106 cells in 3 wells
- DMSO: 2x106 cells in 5 wells
- OLP: 2x106 cells in 5 wells
- Incubate for 20hr in 37oC incubator @ 5% CO2
Day 3: Plate development (20 hours after plating cells)
- Develop IPFL plates the next day (as per manufacturer’s protocol, see next pages)
Ex Vivo 3-color FluoroSpot –Plate prepping (ImmunoSpot)
Materials:
- ImmunoSpot S6 Universal Analyzer
- 3-color FluoroSpot kit (ImmunoSpot)
- IPFL plate (Merck Millipore, Ref# S53J104I07-MB) (comes with the kit)
- 50ml multi-pipette troughs (Optional)
- Multi-channel pipette (Optional)
- Repeater pipette (Optional)
- Syringes
- 22µm filter (Merck Millipore, Ref# SLGP033RS)
- Vacuum manifold or plate washer
- 70% Ethanol (in PBS)
- AIM V medium (Gibco, Ref# 12055-091)
- Blocking solution (AIM V media with 10% KSR)
Day 1: Plate coating
- Prepare coating Abs as per manufacturer’s protocol
- Activate wells by adding 15µl of 70% ethanol for 1 minute at room temperature
- NOTE: Activate all the well even if not be used. Need to activate for vacuum manifold to work. Wells, that’s not bee used for experiments do not let them dry, just add PBS during incubation steps.
- Wash IPFL plate 3x with 200µl sterile PBS (optional: use multi-channel + troughs)
- Plate 80µl of coating Ab solution into each well (optional: use repeater)
- Parafilm (optional) and incubate plate at 4oC O/N
Day 2: Setting up plate
- Flick coating solution, wash 3x with 200µl sterile PBS
- Make blocking solution, AIM V – 10% KSR
- Add 45ml of AIM V to 5ml of KSR
- Add 100µl blocking solution into each well
- Incubate plate at room temperature for at least 30 minutes
- Remove via flicking
- Add PBMCs to the plate after pulsing (as per protocol, see previous page)
- Incubate the plate @ 37oC for 20hr
Day 3: Plate development (20 hours after plating cells)
- Prepare secondary Abs as per manufacturer’s protocol:
- Filtration subtracts 0.5ml from total volume, prepare excess solution as necessary
- Attach 0.22µm filter to syringe; apply and filter solution as per manufacturer’s protocol
- Wash IPFL plate 3x with 200µl PBS
- Add secondary Ab solution, plate 80µl solution into each well
- Incubate plate at room temperature for 2hr in the dark
- Prepare detection Abs as per manufacturer’s protocol:
- Filtration subtracts 0.5ml from total volume, prepare excess solution as necessary
- Attach 0.22µm filter to syringe; apply and filter solution as per manufacturer’s protocol
- Wash IPFL plate 3x with 200µl PBS
- Add detection Ab solution, plate 80µl solution into each well
- Incubate plate at room temperature for 1hr in the dark
- Wash and dry with vacuum manifold using plate washer (necessary step)
- With Microplate washer:
- wash with 200µ PBS and dry via vacuum filtration at -187mmHg for 20s, repeat 4x
- Remove plate underdrain and wash underside with running water, flick and repeat ~2-3x; flick dry as much as possible
- Note: avoid flicking on paper towels to minimize dust/particulates in wells
- Dry ~20-30mins in the BSC (with underside upwards) in the dark
- Scan and count plates, store long-term at 4oC

