Author's Information
Li-Ling Wu 1,2, Hurng-Yi Wang 1, and Pei-Jer Chen 1,3,4
1. Graduate Institute of Clinical Medicine, 2. Department and Institute of Physiology, School of Medicine, National Yang-Ming University, 3. Hepatitis Research Center, and 4. Department of Medical Research, National Taiwan University College of Medicine and National Taiwan University Hospital, Taipei, Taiwan.
Introduction
- The method of hydrodynamic delivery (1, 2) was used to establish a mouse model of hepatitis B virus persistence by transfecting hepatocytes in vivo with HBV genome expressing viral antigens and replicative intermediates, resulting in production of viral particles (3).
- Using the hydrodynamic transfection method, we demonstrated that host genetics and age, and their interaction, are crucial in the generation of strong, diverse immune responses against HBV (4-6). We took advantage of an established HBV-persistence mouse model, by injecting cloned HBV DNA through the tail vein in appropriate, immune-competent mouse strains, to investigate the detailed immune responses against HBV.
- Germ-free animals with hydrodynamic HBV plasmid DNA injection provide an invaluable experimental tool for immunologic role of the gut microbiota in chronic HBV infection, and a direct role for the microbiota in disease development was demonstrated by the use of germ-free mice with HDI.
- This protocol has been previously shown in Hepatitis B Virus: Methods and Protocols-Methods in Molecular Biology (7).
Materials and Reagents
Maintaining GF mouse facility in National Laboratory Animal Center
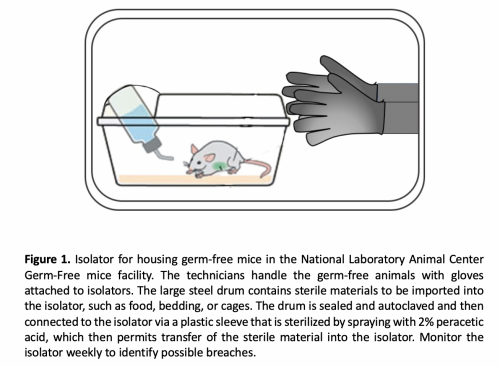
Many mouse strains (specific pathogen free (SPF)or germ-free (GF)) can be used. The protocol describes the details for GF mice that can be adapted to SPF mice. GF mice are produced by hysterectomy reservation, and must be maintained in isolators under very strict handling procedures to keep them germ-free
(Fig1).
GF mouse isolator maintain sterile environment
- Isolators provide physical barriers that allow creation of a sterile environment.
- All manipulation of mice and supplies occurs inside the isolator through gloves and sleeves attached to the isolator walls. In terms of potential contamination, the gloves are most vulnerable, and the most common cause of contaminations are due to holes in the gloves.
Components for preparation of pAAV-HBV1.2 plasmid
- LB medium: Weigh 10 g tryptone, 5 g yeast extract, and 10 g NaCl, and transfer to 2L flask containing 900 mL of water (Note 1). Mix powder well to bring into solution, and transfer the solution to a measuring cylinder and add further water to make a total of 1L. Sterilize at 121 oC for 30 minutes (Note 2). After cooling (to < 50 °C), add ampicillin (stock solution: 50 mg / mL) to the medium making a final concentration of 50 μg ampicillin per mL medium.
Note 1: Having water pre-loaded in the beaker helps to dissolve the components relatively easily, allowing the magnetic stir bar to go to work immediately.
Note 2: Prior to autoclaving, some adjust the pH of LB to desired pH by NaOH or 1 mol/L TRIS stock. We generally did not adjust the pH of LB.
- LB agar: Weigh 5 g tryptone, 2.5 g yeast extract, 5 g NaCl, and 7.5 g agar and transfer to a graduated cylinder containing 250 mL of water (Note 2). Mix powder well to bring into solution, add water to total volume of 500 mL, and transfer to 1 L flask. Sterilize at 121 oC for 20 minutes (making sure to loosen top). Let agar cool to ~55 oC and add 1 mL ampicillin (stock solution: 50 mg / mL). Pour a thin layer (5mm) of LB agar (~10mL) into each plate being careful to not lift the cover off excessively (you should be able to just open up enough to pour). Swirl plate in a circular motion to distribute agar on bottom completely. Let each plate cool until its solid (~20 minutes) then flip so as to avoid condensation on the agar. Store plates in plastic bags in fridge.
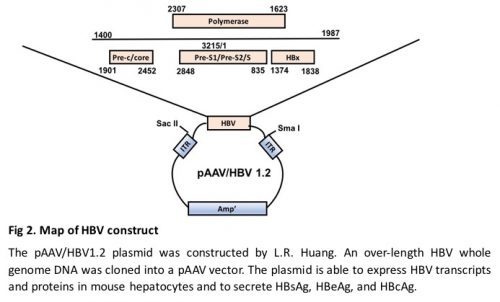
- HBV plasmid, pAAV/HBV1.2, which contains the HBV fragment of genotype A spanning nucleotides 1400--3182/1--1987 by inverted terminal repeats of AAV (Fig. 2), was constructed by previous study (8).
- ECOSTM 101 Competent Cells (Yeastern, Taiwan) (Stored in -80 oC).
- Restriction enzyme: SmaI and SacII (NEB, MA, USA).
- High speed Plasmid Mini Kit (Geneaid, Taiwan).
- EndoFree® Plasmid Maxi kit (QIAGEN, USA).
Hydrodynamic injection material: Prepare all solutions using sterile ultrapure water and analytical grade reagents. Unless otherwise noted, prepare and keep all reagents at room temperature.
- 10X Phosphate buffered saline Buffer, pH7.4 (10x PBS Buffer; containing NaCl 1.37M, KCl 27mM, KH2PO4 18mM, Na2HPO4 100mM).
- 22 µm pore size hydrophilic PVDF membrane (Millex®-GS, Merck Millpore Ltd., USA).
- BD Ultra-Fine™ II Short Needle Insulin Syringe 1 cc 31 G x 8 mm (5/16 in).
- 26-gauge needle and 3 ml syringe
- Ketamine (Imalgene 1000, Merial France).
- Xylazine (Rompun 2%, Bayer German).
- Mouse restraint device
- Heat source (e.g. heat lamp with 120W bulb).
- Alcohol swabs
- GoldenrodTM Animal Lancet 4mm
- Eppendorf tubes
Experimental Procedures
HBV plasmid preparation
- HBV plasmid preparation can start with a competent cell transformation or a glycerol stock (Note 3). Proceed to Step 3, if a glycerol stock is used.
Note 3: A glycerol stock is made from LB culture medium. Add 500 μL of the overnight culture to 500μL of 50% glycerol in a 2 mL screw top tube and gently mix. Store in -80 oC. The 50% glycerol medium is made by diluting 100% glycerol in water. Sterilize the glycerol medium before use.
- Transform ECOSTM 101 Competent Cells with 5 uL HBV plasmid according to manufacturer’s procedure (Note 4). Incubate LB plate at 37 °C overnight.
Note 4: Take one tube of ECOSTM 101 Competent cells out of -80 oC and thaw on ice (~ 20 minutes). Add 5μl of HBV plasmid into competent cells. Vortex for 1 second or flick the bottom of the tube with finger a few times. Place the mixture on ice for 5 minutes. Heat shock the transformation tube by placing the bottom 1/2 of the tube into 42 oC for 45 seconds. Take 100 uL of the competent cell/DNA mixture and plate the LB plate.
- Inoculate the tube containing 5ml LB with a single colony using a pipette tip or with a bacterial stock (Note 5). Incubate with 250 rpm shaking overnight at 37 °C. The tube should be very cloudy as a result of bacteria.
Note 5: To recover bacteria from glycerol stock, open the glycerol stock tube and use a pipette tip to scrape some of the frozen bacteria off of the top. Do not let the glycerol stock unthaw! Dip the pipette tip into LB medium.
- Extract HBV plasmid from 1.5 mL culture medium by High speed Plasmid Mini Kit (Geneaid, Taiwan) according to manufacturer’s procedure (Note 6).
Note 6: High speed Plasmid Mini Kit usually yield 5--10 μg of HBV plasmid dissolved in 50 uL water.
- Check the identity of the extracted plasmid by SmaI and SacII digestion, separately. Follow the procedure provided by the manufacture. Digestion of the HBV plasmid with SmaI will result in two 4 Kb bands. Digestion with SacII will result in a 3.2 Kb and a 4.7 Kb band (Fig. 2).
- Transfer the remaining 3mL culture medium into 1L LB medium. Incubate the medium with 250 rpm shaking at 37 oC for approximately 16 hours.
- Harvest the cells by centrifuging the medium with 6000g for 15min at 4 oC (Note 7) and extract plasmid by using QIAGEN EndoFree® Plasmid Maxi kit.
Note 7: If necessary, make a glycerol stock by acquiring 500 uL culture medium to 500μL of 50% glycerol in a 2 mL screw top tube and gently mix. Store in -80 oC.
- Check DNA quality and concentration by NanoDrop (Note 8). A good quality of plasmid DNA should have A260/A280 ratio ≥ 1.8.
Note 8: 1L LB medium usually yields 800 μg HBV plasmid dissolved in 400 μL water (2μg/μL).
- It is important that the purified plasmid DNA is of high quality and protein-, endotoxin-, DNase-, RNase-free to prevent adverse or toxic effects on the animal. To achieve this, we routinely use the Qiagen Endofree Kit (Qiagen, Cat. #: 12362).
Hydrodynamic Injection
- Measure body weight of the mouse.
- Prepare 10 μg of HBV plasmid DNA dissolved in a volume of PBS equivalent to 8% of the mouse body weight (Note 9). To assist in determining the amount of delivery solution for each individual mouse at the time of injection, it is advised to prepare a worksheet that has already calculated the volume of HBV plasmid needed for each injection and subtracted that volume from the total volume that is to be injected. This allows for the injection procedure to be more time efficient and less prone to error.
| Weight of mouse (g) |
10μg/mL pAAV/HBV 1.2 plasmid DNA concentration (ml) |
A volume of PBS equivalent to 8% of the mouse body weight (ml) |
Total volume
(ml) |
| 20 |
1 |
0.60 |
1.60 |
| 21 |
1 |
0.68 |
1.68 |
| 22 |
1 |
0.76 |
1.76 |
| 23 |
1 |
0.84 |
1.84 |
| 24 |
1 |
0.92 |
1.92 |
Note 9: Make 1x PBS by diluting 10x PBS with water. Sterilize the PBS by autoclave and filter with a 0.22 µm pore size hydrophilic PVDF membrane (Millex®-GS, Merck Millpore Ltd., USA) before use.
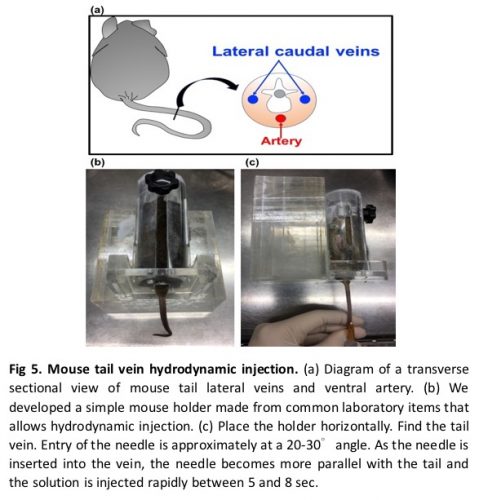
- Dilate the tail vessels prior to injection by warming the mouse tail with a safe and effective heat source (e.g., heat lamp (120W bulb)) for 5-10 minutes (Note 10). This step facilitates tail vein visualization and ensures optimal injection. As the mouse tail warms up, the vein should dilate and become more visible. Keep the mouse warm before hydrodynamic injection (Fig 3).
Note 10: Do not overheat the mouse with the heat lamp. Excessive movement and/or perspiration are indicators of overheating.
- Anesthetize the mouse using Imalgene (ketamine, 60mg/kg) and Rompun™ (xylazine, 12mg/kg) administered by intramuscular injection.
- Secure the mouse with a restraint device before hydrodynamic injection.
- While working under a light source, locate the dilated vein on the ventral side of the mouse tail, preferably near the distal end (tip) of the tail.
- Swab the area with an alcohol pad and allow it air dry to further increase vein visibility and sterilize the site of injection.
- Connect the needle to the syringe and fill with the entire injection solution, ensuring that there is no air bubble in the needle or syringe (Note 11).
Note 11: With the needle pointing up, finger tap the syringe a few times to move air bubbles to the needle and carefully eject the air until a small volume of solution is ejected.
- Place the syringe needle nearly parallel to the tail with the bevel down (toward the tail). Insert the needle into the tail vein (Note 12). If the needle is inserted correctly, the vein should begin to be clear of blood (Note 13).
Note 12: Introduce the needle at the distal portion of the tail. This allows for better observation of the needle entering the vein. If subcutaneous hemorrhaging occurs, the needle can be moved further up (towards the proximal end) to find a new injection site.
Note 13: If the needle is positioned properly upon injection, clearing of the vein will be apparent, and there will be no local swelling or discoloration of the tail. If there is significant resistance, the needle may not be properly inserted into the tail vein. Improper needle insertion into tail tissue is characterized by discoloration and local swelling. When this occurs, remove the needle and reposition it correctly moving further proximal on the tail.
- Dispense the complete volume of the solution into the mouse tail vein within 5--7 seconds at a constant rate (Note 14). A good injection is characterized by a constant resistance that does not increase during the procedure.
Note 14: In our experience, the delivering duration is critical. On the one hand, less than 5 seconds of delivering may increase death rate of the mouse. On the other hand, delivering duration longer than 8 seconds usually compromise the efficacy of HBV transfection.
- Release the mouse from the restraint device.
- Mouse usually tolerates the hydrodynamic injection well, but immediately after injection, it may remain immobile and manifest labored breathing that persists for ~5min. The observed apnea is probably due to a vasovagal response from the large, rapidly administered bolus of HBV DNA solution. Gentle massaging the chest of the mouse is sufficient to stimulate breathing and facilitate recovery (Note 15). The heart rate may slow or increase rapidly within the first minute post injection, however, this should normalize. The mouse should recover within 5 min of the injection. If the mouse appears to be seizing after the injection, this may be an indicator that either an air bubble or an impurity entered the circulation and the mouse may not survive. Careful monitoring of the mice post injection is necessary.
Note 15: Chest messaging decreases the rate of mouse death from roughly 30% to less than 10%. Gently push the chest of the mouse using the index finger at the rate of one time per second for 1 to 2 minutes or until spontaneous breath is recovered. Usually mouse will recover to spontaneous breathing after 1--2 minutes.
- Stop the bleeding by applying the medical gauze to the injection site.
- Collect serum on day 2, 7, 10, and weekly after hydrodynamic injection until the end of experiment (Note 16).
Note 16: There are many ways to collect blood from mice. We use facial vein technique described as follow. Cup the non-dominant hand (the left hand for most people) over the mouse, and scruff it firmly using the thumb and index finger. Locate the hairless freckle on the side of the jaw. Pick up the lancet with your free hand. Point the lancet at the far side of the mouse’s face, at the base of the far ear or at the base of the far side of the mouth. Prick the freckle with the lancet. Collect 4--7 drops of blood to a 1.5 mL eppendorf tube (the amount depends on frequency of bleeding). Release the mouse into its cage when you have obtained your sample. Bleeding should cease immediately.
- Centrifuge the sample for 3 minutes at 16,000 g at room temperature (Note 17).
Note 17: Leaving the blood sample untreated longer than a couple of hours is not recommended.
- Transfer the supernatant into a new eppendorf and repeat Step 14 to obtain serum. The serum is ready for analysis. If necessary, store at -20 o
- Measure HBsAg and anti-HBs using an ABBOTT ARCHITECT HbsAg and anti-HBs kits (ARCHITECT I system) (Note 18) (Note 19).
Note 18: Specimens with an HBsAg value exceeding 250 IU/mL, are flagged with the code “>250.00 IU/mL”. Nevertheless, usually serum HBsAg will reach to highest level, which is several to 10 thousands IU/mL, between 2 to 7 days after hydrodynamic injection, and the value will drop gradually afterwards. We will make 1:40 dilution by adding 10 μL serum into 390 μL PBS during the first 2 weeks after hydrodynamic injection. After that, the dilution factor will become 1:20.
Note 19: In our experience, mice with serum HBsAg lower than 600 IU/mL at 2 days after HDI is a sign of unsuccessful transfection and will not be included for further follow-up.
Conclusion and future prospects
As described above, hydrodynamic injection provides an effective approach to deliver DNA, RNA, protein and virus into hepatocytes. Besides the applications in the studies of liver diseases, HDI by HBV DNA in either SPF or GF mice provide a convenient and invaluable experimental tool for studying immunology of hepatitis B and also the role of the gut microbiota. Such research outcomes will continue to advance to benefit both basic and clinical research of hepatitis B.
Acknowledgement
Patient Derived Tumor, Gut Microbiota Transplantation and Gene Modification Service at the National Core Facility for Biopharmaceuticals (MOST 107-2319-B-492-001), National Laboratory Animal Center, National Applied Research Laboratories, Taiwan. This work was supported by MOST grant 107-2321-B-002 -002 –, 107-2811-B-002 -582 - and 107-2321-B-002 -004 -for the Advancement of science and technology to RHS.
References
- Zhang G, Budker V, Wolff JA. High Levels of Foreign Gene Expression in Hepatocytes after Tail Vein Injections of Naked Plasmid DNA. Human Gene Therapy 1999;10:1735-1737.
- Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6(7):1258-1266.
- Yang PL, Althage A, Chung J, Chisari FV. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc Natl Acad Sci U S A 2002;99:13825-13830.
- Wu LL, Peng WH, Wu HL, Miaw SC, Yeh SH, Yang HC, PH L, et al. Lymphocyte Antigen 6 Complex, Locus C+ Monocytes and Kupffer Cells Orchestrate Liver Immune Responses Against Hepatitis B Virus in Mice. Hepatology, 1-17 [Epub ahead of print] 2019.
- Chou HH, Chien WH, Wu LL, Cheng CH, Chung CH, Horng JH, Ni YH, et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc Natl Acad Sci U S A 2015;112:2175-2180.
- Ebert G, Preston S, Allison C, Cooney J, Toe JG, Stutz MD, Ojaimi S, et al. Cellular inhibitor of apoptosis proteins prevent clearance of hepatitis B virus. Proc Natl Acad Sci U S A 2015;112:5797-5802.
- Wu LL, Wang HY, P.J. C. Hydrodynamic HBV Transfection Mouse Model. Hepatitis B Virus Methods and Protocols-Methods in Molecular Biology. New York, USA: Springer New York. 2016:227-235.
- Huang L-R, Wu H-L, Chen P-J, Chen D-S. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc Natl Acad Sci U S A. 2006;103:17862-17867.