Experimental Procedures
- Stem cells culture and passage
Routine non-colony-type culture of human pluripotent stem cells (hPSCs)
- Colony type cultures of hPSCs (hESC or hiPSC) were adapted to non-colony monolayer type culture as described previously by Chen et al. (Chen KG, et al. Stem Cell Research 2012).
- Once adapted to non-colony type culture, hPSCs are routinely maintained in 6-well plate containing 2 ml of mTeSR1, previously coated with high-concentrated growth factors reduced (GF (-)) Matrigel (0.4 mg/ml).
- On the day cells reach confluence, new plates are coated with high concentrated GF (-) Matrigel.
- 250 µl of GF (-) Matrigel are resuspended in 6 mL of DMEM-F12 (Final concentration: 0.4 mg/ml), and allowed to rest for 1 hour on ice for homogenization.
- 1 mL of the high concentrated GF (-) Matrigel solution is transferred to each well of a 6 well plate.
- The plate is incubated at 37°C for 15 minutes and then sealed for 4°C storage.
- The day after, polymerized Matrigel should be visible at high magnification (x40) using a contrast phase microscope.
- The overconfluent hPSCs, ready for passage, are washed once with PBS.
- Cells are incubated with 1 mL of gentle dissociation buffer (Accutase, Life Technologies) at 37°C for 5-8 minutes.
- Cells are resuspended in 9 mL of mTeSR1 medium.
- At this point, 50 µL of cell suspension can be collected for cell count.
- Centrifugation 3 minutes at 2,000 rpm at RT
- Supernatant is discarded.
- The pellet is resuspended in mTeSR1.
- Cells are passed 1/4 to 1/5 on high-concentrated GF (-) Matrigel-coated plates in mTeSR1 + 10 µM of Rock Inhibitor.
- Cells are cultured at 37°C, 5% CO
2, normal O
2, with mTeSR1 medium changed daily.
Note: Different clones of human stem cells (either iPSC or ESC) may differentiate differently to hepatocyte-like cells, which may also have different infectious efficiency by HBV. It is recommended to test various human stem cell lines to obtain the optimal lines for differentiation and infection. See attached references for lines we have used successfully for this purpose. We will be depositing our stem cell clones to the HBV Resource Repository.
- Hepatocyte-like cells differentiation
Passing hPSCs for differentiation (timing: 1 day)
Day -1: Passage hPSCs in 6 well plate for definitive endoderm induction
- Pre-coating of 6 well plate with low concentration GF (-) Matrigel: 250 µL of GF (-) Matrigel (Corning) in 20 mL of DMEM-F12 (0.125 mg/mL)
- Allow to rest 1 hour on ice to ensure homogenization.
- Distribute 1 mL per well of a 6 well plate.
- Incubate at least 15 minutes at 37°C for polymerization.
- hESCs or hiPSCs are washed with 2 mL PBS at RT.
- Add 1 mL of gentle dissociation buffer (Accutase, Life Technologies) per well, 5-8 minutes at 37°C.
- Resuspend in 9 mL of mTeSR1.
- Count cells with trypan blue exclusion test.
- Centrifuge 3 minutes at 2000 rpm at RT.
- Discard supernatant.
- Resuspend cell pellet at a concentration of 1 millions cells per mL + 10µM of Rock Inhibitor.
- Transfer 2 mL (2 millions cells) per well of a 6-well plate, previously coated with low concentrated GF (-) Matrigel.
- Incubate the cells at 37°C, 5% CO
2, normal O
2, overnight.
Definitive endoderm induction (timing: 4 days)
Day 0: Definitive endoderm induction (day 1, D1) Supplement MR and CJ
- Cells seeded the day before should cover more than 90% of the well.
- 1 wash with PBS at RT to discard dead floating cells.
- Add 2 mL of STEMCELL Technologies Definitive Endoderm Basal Medium + 20 µL of supplement MR + 20 µL of supplement CJ per well.
- Incubate overnight at 37°C, 5% CO
2, normal O
2.
Trouble shooting:
- Cells are not confluent: Seed 2 million living cells at day 0, in presence of 10 Mm Rock Inhibitor. Ensure high quality of pluripotent stem cells.
Day 1: Definitive endoderm induction (D2) Supplement CJ
- Gently shake the plate to float dead cells and discard medium.
- Add 2 mL of Basal Medium + 20 µL of supplement CJ per well.
- Incubate at 37°C, 5% CO
2, normal O
2.
Day 2: Definitive endoderm induction (D3) Supplement CJ
- Discard medium.
- Add 2 mL of Basal Medium + 20 µl of supplement CJ per well.
- Incubate at 37°C, 5% CO
2, normal O
2.
Day 3: Definitive endoderm induction (D4) Supplement CJ
- Discard medium.
- Add 2 mL of Basal Medium + 20 µl of supplement CJ per well
- Incubate at 37°C, 5% CO
2, normal O
2.
Day 4: Assessment of definitive endoderm (=DE) induction
- At that point, cells should cover the entire surface of the wells, between 3 and 3.5 millions cells per well of a 6 well plate.
- DE cells can be assessed for expression of definitive markers SOX17 and FoxA2, by immunofluorescence(IF) (Fixation with PFA 4%) or FACS analysis (after resuspension with Accutase).
Trouble shooting:
- Cells are not confluent: May indicate higher susceptibility to cell death during supplement MR treatment.
- Cells are negative for SOX17/FoxA2: Validate quality of stem cell population used (IF for pluripotency markers).
Hepatic specification (timing: 8 days)
Day 4: Passage for Hepatic Specification
- Plates used for the hepatic differentiation must be coated with low concentration GF (-) Matrigel (0.125 mg/mL) as described on day -1
Format Surface Volume of Matrigel per well
6-well plates: 9.5 cm
2 1 mL
12-well plates: 3.8 cm
2 0.5 mL
24-well plates: 1.9 cm
2 0.25 mL
96-well plates 0.32 cm
2 60 µL
384-well plates 0.056 cm
2 40 µL
- Discard supernatant.
- Wash cells once with RT PBS.
- Add 1 mL of Accutase per well.
- Incubate 5-8 minutes at 37°C.
- Resuspend in 9 mL of Differentiation Medium.
Differentiation Medium:
DMEM (4.5 g/L glucose) 225 mL
F12 medium 225 mL
Knock Out Serum Replacement 50 mL
NEAA 5 mL
Penicillin Streptomycin 5 mL
Glutamine 5 mL
- Use 50 µL for cell count with trypan blue exclusion test.
- Centrifuge at 2000 rpm, 3 minutes at RT
- Supernatant is discarded.
- Cell pellet is resuspended in differentiation medium + 100 ng/mL of HGF + 1% DMSO + 10 µM Rock Inhibitor, at a concentration depending on the well format chosen for subsequent hepatic specification.
- The cell suspension is then distributed in the well pre-coated with low concentration GF (-) Matrigel.
- Concentration of cells for hepatic specification can vary from cell line to cell line, but we suggest a starting concentration of 79.000 cells/cm
2.
Format Surface Suggested concentration Volume per well
6 well plates: 9.5 cm
2 750.000 cells per well 2 mL
12 wells plates: 3.8 cm
2 300.000 cells per well 1 mL
24 wells plates: 1.9 cm
2 150.000 cells per well 0.5 mL
96 wells plates 0.32 cm
2 25.000 cells per well 100 µL
384 wells plates 0.056 cm
2 4.400 cells per well 40 µL
- Cells are then incubated overnight at 37°C, 5% CO
2, normal O
2.
Day 5: Hepatic Specification Day 2
- At that time, most of the cells should be adherent, covering around 75% of the well surface.
- Cells are washed once with PBS.
- Add 2 mL of Differentiation medium + 100 ng/mL HGF + 1% DMSO per well.
- Incubate the cells at 37°C, 5% CO
2, normal O
2.
Trouble shooting:
Cells are non-adherent: On day 4, ensure the extracellular matrix is polymerized. Polymerized Matrigel should be visible using an inverted contrast phase microscope at high magnification (>20x). Do not forget Rock Inhibitor at the time of seeding.
Day 6-7 : Hepatic Specification Day 3-4
- Daily replace the Differentiation medium + 100 ng/mL HGF + 1% DMSO
- Incubate the cells at 37°C, 5% CO
2, normal O
2.
Day 8-11: Hepatic Specification Day 5-8
- At that time, the cells should be confluent, and be growth-arrested.
- Daily replace the Differentiation medium + 100 ng/mL HGF + 1% DMSO
- Incubate the cells at 37°C, 5% CO
2, normal O
2.
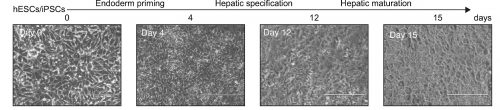
Day 12: Assessment Hepatic specification
- Cells at day 12 can be assessed for expression of hepatoblast markers, like the Hepatocyte Nuclear Factor 4 alpha (HNF4A), a master regulator of hepatic differentiation, and alpha-fetoprotein (AFP), by immunofluorescence or FACS analysis.
- AFP secretion can be assessed by ELISA on supernatant of culture collected on day 12.
Trouble shooting:
- Cells are not confluent: Seed more cells on day 4
- Cell population is very heterogeneous: validate quality/homogeneity of the DE cell population before plating.
- Cells are negative for AFP: Check quality/lot/concentration of HGF.
Hepatic maturation (timing: 3 days)
Day 12-14: Hepatic Maturation Day 1-3
- Supernatant is discarded.
- Daily replace by Differentiation medium + 10
-7 M Dexamethasone.
Day 15: Assessment of Hepatic Maturation
- At that time, the cell morphology should be similar or close to the one of PHHs: Polygonal cells, tight junctions, small round nuclei, sometimes binucleated cells, and lipid droplets visible in the cytoplasm.
- Expression of hepatic marker, for example albumin (ALB) , alpha-fetoprotein (AFP) , alpha-1-antitrypsin (AAT) can be assessed by IFA. Secretion of AFP or ALB can also be assessed by ELISA on supernatant of differentiated cells.
Trouble shooting:
- Cells not confluent at day 15: Seed more cells on day 4. Check that cells were seeded in presence of Rock Inhibitor. Monitor cell death (floating cells) to ensure viability of the cells.
- Cells too confluent / multilayered at day 15: Ensure the quality/homogeneity of the DE cells, as the DE-derived hepatoblasts should be growth-arrested. Cells on the top layer are usually non-directed differentiation from non-DE cells cultured in presence of DMSO. Seed less DE cells per cm
2 on day 4.
- Cells are not polygonal: Assess expression of AFP to validate the hepatic specification. If cells are still AFP positive, it means the hepatic maturation didn’t occur. Check quality/concentration/lot of dexamethasone.
- Cells are negative for ALB but positive for AFP/HNF4A: Check dexamethasone, ensure good cell density. If cells not confluent, seed more cells on day 4.
- HBV infection
Cell culture derived HBV was concentrated from the supernatant of HepG2.2.15 cells using centrifugal filter devices (Centricon Plus-70, Biomax 100.000, Millipore Corp., Bedford, MA) and titered by HBV DNA qPCR. Immediately after collection, the virus stock was divided in aliquots and stored at -80°C until use. For infection, inoculation of cells was performed with multiplicity of infection (MOI) 200-300 in WEM medium containing 5% PEG 8000 (Sigma Aldrich, St. Louis, USA) for 16 h. At the end of incubation period, HLCs were washed three times with PBS and cultured in WEM medium.
MAINTENANCE OF HLCs (TIMING: 0-3 WEEKS)
At that point, differentiated HLCs can be kept more than 2 weeks in the Hepatocyte Maintenance Medium.
Hepatocytes Maintenance Medium:
William’s E Medium 500 mL
FBS 50 mL
Penicillin Streptomycin 5 mL
Human insulin 1 µg/mL (350µL)
Hydrocortisone 5 µg/mL
DMSO 1.8%
The medium should be changed every 2-3 days.
At that stage, the cell morphology should continue toward a more mature hepatic phenotype.
Trouble shooting:
- Cell death: Ensure the right concentration of DMSO, as too high concentration may exert a cytotoxic effect on the cells.